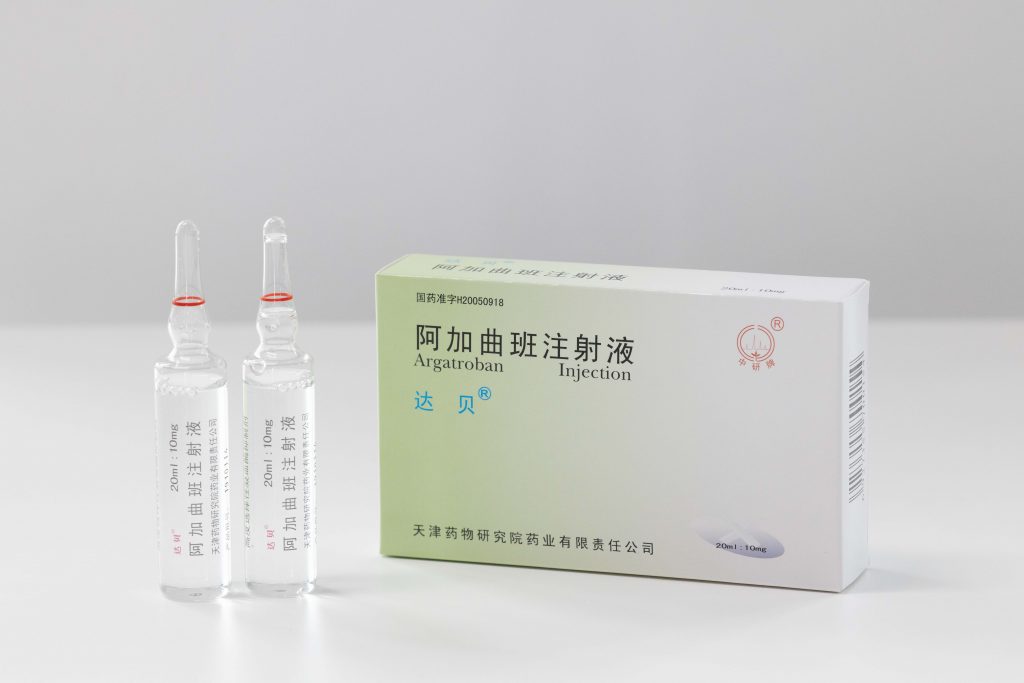
Looking into the future, Tianjin Institute of Pharmaceutical Research will bring into full play to its own superiority, proactively attract and train talents, make contribution to building a world-class pharmaceutical research institute and a century-old pharmaceutical research institute, and endeavor to stride forward towards a domestic leading and international excellent pharmaceutical enterprise.